electron configuration of pd|electron configuration calculator : Baguio You can write the electron configuration using the Aufbau principle triangle: However, the experiment has shown that the electron configuration of Palladium is: 46P .
The Pennsylvania Lottery 1200 Fulling Mill Road, Suite One, Middletown, PA 17057 Call: 1-800-692-7481 | More Contact Options
PH0 · pd+2 electron configuration
PH1 · pb 4+ electron configuration
PH2 · electron configuration guide
PH3 · electron configuration generator
PH4 · electron configuration for every element
PH5 · electron configuration chart pdf
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa
The best handball betting sites provide a live streaming service for many European leagues. Alternatively, you can follow the end-to-end action by watching a graphical representation of the match. Most Popular Handball Betting Markets. There aren’t many handball betting markets when you compare it with other sports. Although you won’t .
electron configuration of pd*******The ground state electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. This electron configuration shows that the d-orbital has a total of ten electrons. Therefore, the valence electronsof palladium are ten. The elements that form bonds by donating electrons are called cation. . Tingnan ang higit paThe total number of electrons in palladium is forty-six. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in palladium in specific rules in different orbits and orbitals is called the electron . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit pa
The simplified or abbreviated Electron configuration of palladium is [Kr]4d10. This element is composed of 46 electrons, their distribution is as follows: in the first layer there are 2 .
You can write the electron configuration using the Aufbau principle triangle: However, the experiment has shown that the electron configuration of Palladium is: 46P .
You can write the electron configuration using the Aufbau principle triangle: However, the experiment has shown that the electron configuration of Palladium is: 46 P d: 1 s 2, 2 s .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
The chemical symbol for Palladium is Pd. Electron Configuration and Oxidation States of Palladium. Electron configuration of Palladium is [Kr] 4d10. Possible .
Palladium, with its atomic number of 46, has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d10. Understanding the electron configuration helps us .
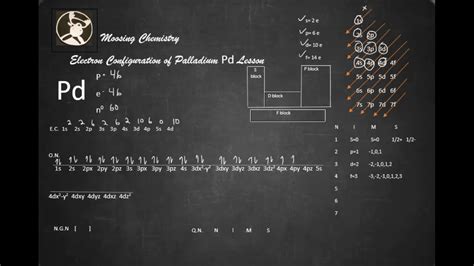
Now, The electron configuration of Pd is: [Kr] 4d 10 and The electron configuration of Pd 2+ is: [Kr] 4d 8. So, in this most common oxidation state of palladium (Pd 2+), if we see the electron . The electronic configuration notation of Pd is written as [Kr] 4d 10, where Kr represents the electronic configuration of krypton as a noble gas which is 36, followed by . Palladium Electron Configuration: Electron configuration is conducted having a chemical element in which the distribution of the electrons for that particular chemical item is made for the orbital or the .
electron configuration calculatorIn this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .
In this video, we’ll discuss this in more depth and walk through all of the electron configurations for the 3d transition metals.. Created by Jay. Questions Tips & Thanks. . as . In this way, the electrons of an atom, molecule, or ion harmonize into the most stable electron configuration possible. Electron behavior is elaborated by other principles of atomic physics, such as Hund's rule and the Pauli exclusion principle. Hund's rule asserts that even if multiple orbitals of the same energy are available, electrons fill .electron configuration of pd electron configuration calculator Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.Palladium is a chemical element of the periodic table with chemical symbol Pd and atomic number 46 with an atomic weight of 106.421 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: Electrons per shell: 2, 8, 18, 18: Valence electrons : 10: Valency electrons : 4:Orbital diagram. Palladium electron configuration. Pd (Palladium) is an element with position number 46 in the periodic table. Located in the V period. Melting point: 1552 ℃. Density: 12.02 g/cm 3 . The order of filling the orbitals with electrons in the Pd atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 .Here [Ne] refers to the core electrons which are the same as for the element neon (Ne), the last noble gas before phosphorus in the periodic table. The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. The Palladium element has the 46 electrons you can refer to the periodic table to calculate that and we further know about the S orbital, which can only retain the maximum 2 number of electrons. Further P can hold 6, d can hold 10 and the f can at last hold 14. With this equation the electron configuration becomes as 1s 2 2s 2 2p 6 3s 2 . Hund's Rule. Hund's rule suggests that electrons prefer parallel spins in separate orbitals of subshells. This rule guides us in assigning electrons to different states in each sub-shell of the atomic orbitals. In other words, electrons fill each and all orbitals in the subshell before they pair up with opposite spins.
Palladium, symbolized by Pd, is a silvery white metal that possesses unique electron configurations and atomic structures. In this section, we will delve into the electron configuration of palladium and explore its atomic properties. Key Takeaways: The electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d10.
An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or .Element Palladium (Pd), Group 10, Atomic Number 46, d-block, Mass 106.42. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.Thus, Pd's configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ (the 5s is empty) . And so for an electron configuration for the elements in the third period, so this would be the first period, second period, the third period. So let's do sodium. Sodium has 11 electrons so one more than neon but the second shell is full .electron configuration of pd Now, The electron configuration of Pd is: [Kr] 4d 10 and The electron configuration of Pd 2+ is: [Kr] 4d 8. So, in this most common oxidation state of palladium (Pd 2+), if we see the electron configuration, then it possesses incomplete d-orbitals. The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .

Electron Configuration Palladium The element Palladium is an exception to the standard diagonal rules.Palladium has 46 electrons and does not have a 5s orbit.
Condensed electron configuration Write the condensed electron configuration for the following pairs: a. Mn atom b. Mn^2+ c. Sb atom d. Sb^3-Write the ground state electron configuration for palladium (Pd). Write the expected electron configuration for Na. Write the electron configuration and shorthand electron configuration of Sr2+.
Daylight Saving: This is a standard time zone, however during summer some places switch clocks for one hour forward when daylight saving comes into effect and observe Eastern European Summer Time (EEST). End: Eastern European Time (EET) has ended on Sunday, March 31, 2024 at 3:00 am local time and clocks were set one hour forward to .
electron configuration of pd|electron configuration calculator